12 Panel Drug Screen
£8.99 Original price was: £8.99.£6.99Current price is: £6.99.
- Tests for 12 common illegal drugs with over 99% accuracy
- Easy, fast results in minutes by dipping test in urine sample
- Two line result indicates negative, one line means positive
- FDA approved and CLIA waived for over-the-counter use
- Ideal for home use, workplaces, schools.
In stock
For individuals seeking an all-encompassing drug test that checks for the most frequently abused substances, this 12 panel urine test is an optimal and affordable option. With the ability to detect twelve of the most commonly used illegal drugs in one simple test, this kit offers a thorough and convenient screening method.
FDA Approved and CLIA Waived for home or over-the-counter (OTC) use.
Results from this test can be obtained in just 5 minutes.
Long Expiry Dates
These easy to store and use drug screen tests have a long expiry date, meaning that they are ideal to keep on the shelf for regular drug testing and monitoring.
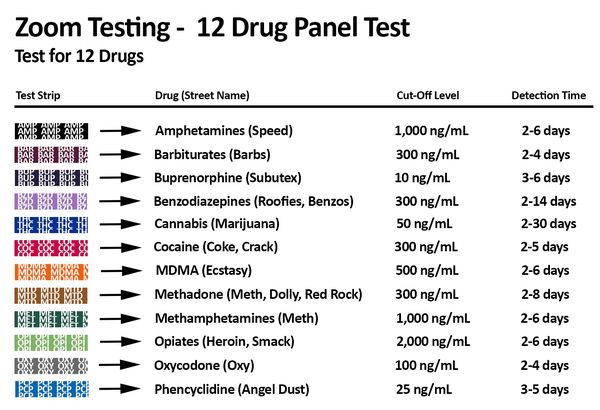
This 12 Panel Drug Screen screens simultaneously for the following drugs:
-
- AMP Amphetamines (1000 ng/ml)
- BAR Barbiturates (300 ng/ml)
- BUP Buprenorphine (10 ng/ml)
- BZD Benzodiazepines (300 ng/ml)
- THC Cannabis (50 ng/ml)
- COC Cocaine (300 ng/ml)
- MDMA Ecstasy (500 ng/mL)
- mAMP / MET Methamphetamines (1000 ng/ml)
- MTD Methadone (300 ng/ml)
- MOP / OPI Opiates (Heroin) (2,000 ng/ml)
- OXY Oxycodone (100 ng/ml)
- PCP Phencyclidine (25 ng/ml)
What does FDA Approved mean in relation to this drug test?
The term “FDA Approved” means this drug test kit has been reviewed and approved by the U.S. Food and Drug Administration. This means the FDA has determined the test is reasonably safe and effective for its intended use, when used according to the instructions.
What does CLIA Waived mean in relation to this drug test?
The term “CLIA Waived” means the test has been deemed simple and accurate enough that it can be used in non-laboratory settings. CLIA refers to the Clinical Laboratory Improvement Amendments, which regulate testing on humans.
Waived status means the test is very simple to use and has a low risk of giving incorrect results. Tests that are CLIA waived can be sold over-the-counter (OTC) for use at home or in offices, clinics, etc. They don’t require being administered by trained lab personnel.
Related Products
This site uses Akismet to reduce spam. Learn how your comment data is processed.






Reviews
There are no reviews yet